VISION
To be a reliable pharmaceutical packaging company for global clients
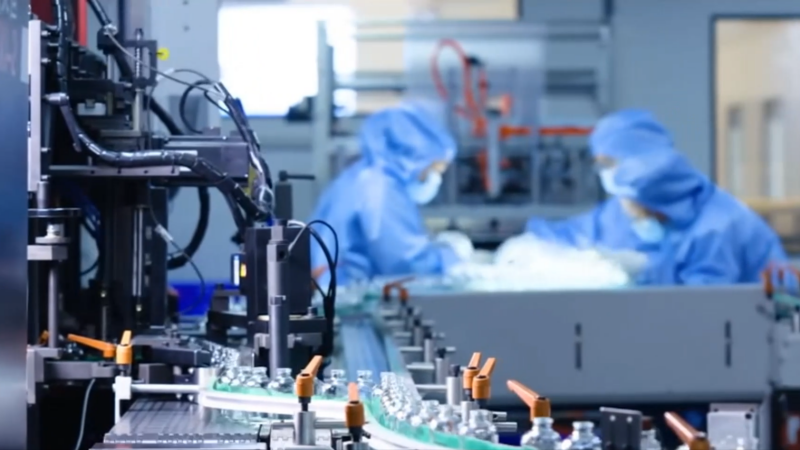
Shandong Linuo Pharmaceutical Packaging Co., Ltd. was founded in 1995 and has over 30 years of experience providing high-quality pharmaceutical glass packaging. We specialize in borosilicate ampoules, injection vials, molded glass bottles, and cartridges, serving more than 10,000 clients in over 100 countries and trusted by 1 in 3 pharmaceutical companies worldwide.
With headquarters in Jinan, Shanghai, and Dubai, R&D centers in Germany and Japan, and production bases across the Middle East, Southeast Asia, and South America, Linuo combines global reach with a resilient, fully integrated supply chain. Our annual capacity exceeds 9.6 billion units, supported by $694M+ in annual sales, 500+ advanced production machines, and smart factories spanning 900,000+ m².
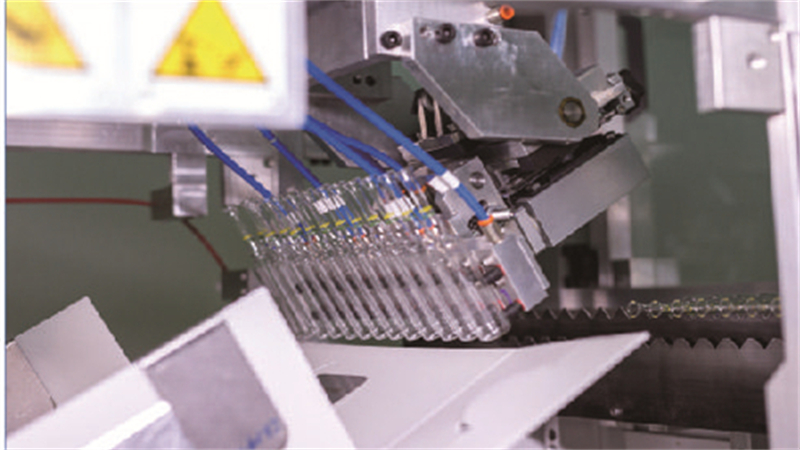
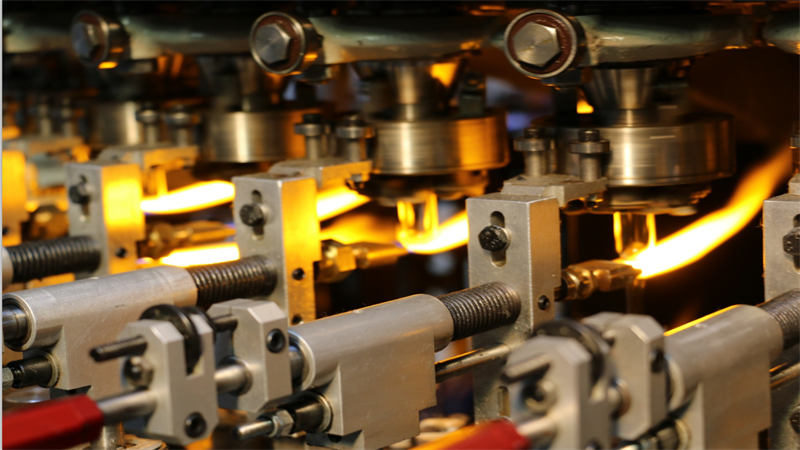
Innovation and quality are at the core of our operations. With 130+ patented technologies, $4.2M+ in annual R&D, ISO certifications, FDA DMF, EU CEP, and CNAS-accredited labs, we provide custom solutions across performance, form, printing, sterilization, and packaging. Our smart manufacturing uses AI inspection, robotics, digital twin technology, and “Zero Glass Debris” systems to ensure safety and consistency.
Committed to sustainability, ESG principles are integrated across our operations and product lifecycle. Linuo is dedicated to being a global leader in pharmaceutical glass packaging, delivering safer, smarter, and more reliable solutions to the healthcare and medical industries worldwide.
Milestones of Development

1995
Linuo was established in Yuhuangmiao Town, Jinan.

2012
A laboratory accredited by CNAS

2021
Went public under the stock code 301188

2024
Launched overseas strategic investment
Why Choose Linuo
Reliable Quality & Excellent Service
With superior technological strength and extensive industry experience, Linuo provides high-quality pharmaceutical glass packaging products and services to global customers.
30+ years of global service provision: Focusing on pharmaceutical glass packaging material research and production for 30 years
Certifications:
Certified with ISO15378, ISO9001, ISO14001, ISO45001, ISO50001, and other certification systems for pharma glass packaging products
Environmental commitment:
We are committed to being a green and environmentally friendly enterprise
Compliance with international pharmacopoeias:
Comply with EP, USP, JP, and Chinese Pharmacopoeia
FDA DMF registration number:
- Ampoules: 27095;
- Injection vials: 27096;
- Molded glass bottles: 38426;
- Cartridge: 40598;
- RTU Vials & Cartridge: 040947
CNAS laboratory accreditation:
Our laboratory is accredited by the China National Accreditation Service for Conformity Assessment (CNAS L5691)
Certifications